Instruments

Bruker 600 MHz AVANCE III NMR Spectrometer with Cryoprobe
- Optimized for very high 13C sensitivity and very good 1H performance
- Operates with a 5mm cryogenic probe
- Includes a sample case that holds 24 samples
- Has temperature range limits for data collection ranging from 10°to 50°C
- Has remote data collection capabilities. Please email Hyun Lee (danielhl@uic.edu) if you would like to have remote data collection capability
- Reservation: available 24 hours/day Mon-Sun
- Minimum reservation: 30 minutes
- Time increment: 30 minutes
- Maximum reservation at a time: 48 hours
Instruments

Beamline 21-ID-D
- Fully tunable beamline (6–20 keV) with Kohzu monochromator and bimorph mirrors
- Has a new DECTRIS EIGER2 16M
- The flux on 21-ID-D is roughly an order of magnitude stronger than the side stations, necessitating the EIGER2 ability for dual readout to prevent saturation
Beamline 21-ID-F & Beamline 21-ID-G
- Fixed-wavelength stations (~0.9786 Å) above the Se peak and ideal for routine SAD
- All three stations are being upgraded to have Arinax MD3-UP microdiffractometers along with Pixel Array Detectors
- 21-ID-F a DECTRIS EIGER 9M, and 21-ID-G has a DECTRIS PILATUS 6M on loan from the original SBC-CAT, Sector 19.
- New sample changers will hold 37 UniPucks and eventually support fully automated data collection.
Beamline Status Update
- 21-ID-G is currently available for remote and on-site collection with the original CATS sample changer supporting SPINE baskets and a MarMosaic 300 CCD detector
- 21-ID-F is available for on-site collection with an EIGER 9M PAD
- 21-ID-D is largely dismantled during its upgrade this summer with some serial crystallography experiments being performed in parallel
Additional Services
600 NMR Data Acquisition
600 NMR Data Acquisition Service by staff is available.
Training
- Bruker 600 MHz NMR for new users
- Bruker 600 MHz NMR for COP or Chemistry NMR users
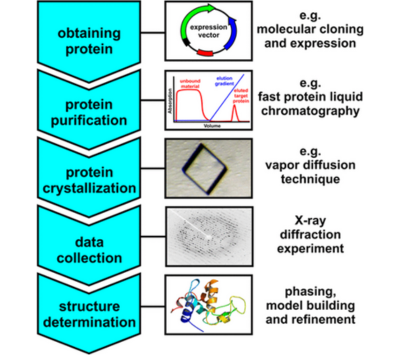
X-ray Crystallography
The Structural Biology Core supports research projects requiring macromolecular structure determination and biophysical characterization through X-ray crystallography. Investigators may submit concentrated protein samples (10–15 mg/mL) for crystallization screening. If protein purification is also needed, please submit a “Protein Purification Service” request through the Biophysics Core on iLab, providing detailed information and specifying your starting point for the X-ray crystallography workflow.
Please note that data analysis and sample setup timelines may vary significantly depending on sample quality and project complexity. For consultation, please contact Dr. Hyun Lee (danielhl@uic.edu) or Val Yu (qihyu@uic.edu).